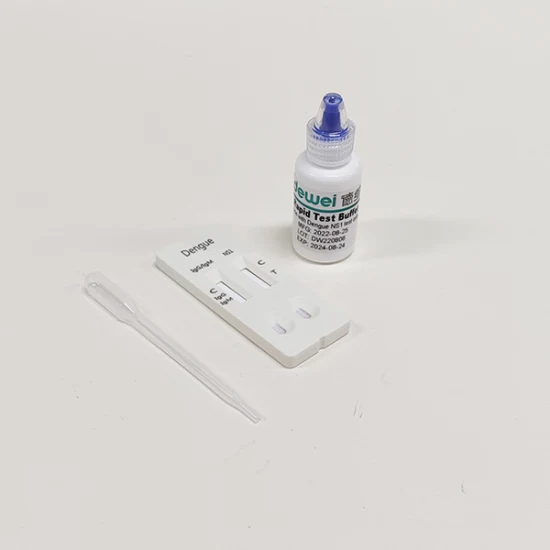
Dengue Rapid Test Diagnostic Kit of Ns1 Antigen or Igg/Igm Antibody Combo
Basic Info.
| Model NO. | DEN |
| Result Time | Within 15 Mins |
| Transport Package | 40tests/Box |
| Specification | 40tests/box |
| Trademark | Dewei |
| Origin | China |
Product Description
INTENDED USE
The Dengue Rapid Test Cassette (Whole Blood/Serum/Plasma) is a rapid chromatographic immunoassay for the qualitative detection of IgG and IgM antibodies to Dengue virus in human whole blood, serum, or plasma as an aid in the diagnosis of primary and secondary Dengue infections.
| Storage | 2~30 º C |
| Specimen | Whole Blood/Serum/Plasma |
| Component | Rapid Test + Buffer + Pipettes |
| Principle | Colloidal gold rapid tests |
| Reading | Within 15mins |
| Package | 40tests/box |
| Trademark | Dewei |
| Origin | China |
INTRODUCTION
Dengue is a flavivirus, transmitted by Aedes aegypti and Aedes albopictus mosquitoes. It is widely distributed throughout the tropical and subtropical areas of the world,1 and causes up to 100 million infections annually. Classic Dengue infection is characterized by a sudden onset of fever, intense headache, myalgia, arthralgia and rash. Primary Dengue infection causes IgM antibodies to increase to a detectable level in 3 to 5 days after the onset of fever. IgM antibodies generally persist for 30 to 90 days. Most Dengue patients in endemic regions have secondary infections, resulting in high levels of specific IgG antibodies prior to or simultaneous with IgM response. Therefore, the detection of specific anti-Dengue IgM and IgG antibodies can also help to distinguish between primary and secondary infections.
The Dengue Rapid Test Device (Whole Blood/Serum/Plasma) is a rapid test that utilizes a combination of Dengue antigen coated colored particles for the detection of IgG and IgM Dengue antibodies in human whole blood, serum, or plasma.
MAIN CONTENTS
• A reaction test Cassette with desiccant.
• Buffer
• Disposable pipettes.
• Instructions for use.
STORAGE AND STABILITY
• Store at 2 ~ 30 º C in the sealed pouch for 24 months.
PRECAUTIONS
• For in vitro diagnostic use only.
• Do not use after expiration date.
• The test Cassette should remain in the sealed pouch until use.
• The used test Cassette should be discarded according to local regulations.
DIRECTION OF USE
Allow the test device, specimen, buffer, and/or controls to reach room temperature (15~30°C) prior to testing.
1. Bring the pouch to room temperature before opening. Remove the test device from the sealed pouch and use it as soon as possible.
2. Place the test device on a clean and level surface.
For Serum or Plasma Specimens:
Hold the dropper vertically, draw the specimen up to the Fill Line (approximately 5 uL), and transfer the specimen to the specimen well (S) of the test device, then add 3 drops of buffer (approximately 90 mL) and start the timer. See illustration below. Avoid trapping air bubbles in the specimen well (S).
For Whole Blood (Venipuncture/Fingerstick) Specimens:
To use a dropper: Hold the dropper vertically, draw the specimen 0.5-1 cm above the Fill Line, and transfer 1 drop of whole blood (approximately 10 µL) to the specimen well (S) of the test device, then add 3 drops of buffer (approximately 90 uL) and start the timer. See illustration below.
To use a micropipette: Pipette and dispense 10 µL of whole blood to the specimen well (S) of the test device, then add 3 drops of buffer (approximately 90 µL) and start the timer. See illustration below.
3. Wait for the colored line(s) to appear. Read results at 10 minutes. Do not interpret the result after 20 minutes.
INTERPRETATION
POSITIVE: Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T).
NEGATIVE: Only one colored band appears, in the control region (C). No apparent colored band appears in the test region (T).
INVALID: Control band fails to appear. Results from any test which has not produced a control band at the specified read time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor.PRODUCT IMAGES
R&D TEAMPRODUCTION LINECERTIFICATESEXHIBITIONS SHIPMENTS